#17: Why we didn't get a malaria vaccine sooner (bonus content)
This week: How to write a 9,000+ word article, fact-checking and history heuristics, and the path to scientific discovery.
This is my seventeenth post of Scientific Discovery, a newsletter where I’ll share great new scientific research that you may have missed. Check out the About page if you’re interested in why I’m writing this.
I’ve been holed up over the last few months co-authoring a long piece called ‘Why we didn’t get a malaria vaccine sooner’ with two fantastic economists, Rachel Glennerster and Siddhartha Haria. It’s the lead piece in the latest issue of Works in Progress.
If you haven’t read it already, I think you’ll really enjoy it:
The response so far has been incredibly positive and we’re very grateful that so many people read, enjoyed and learnt from it. Some very kind reviews so far: a ‘phenomenal writeup of the history of malaria vaccine efforts’, according to Alexander Berger, an ‘incredible read on the history, science, economics, and politics involved’, from Shruti Rajagopalan, and ‘just as good as everyone is saying: a deep, hugely informative story’, from Stuart Ritchie. Thank you!
Here’s my favourite chart I made for the piece. It’s a timeline to show how many vaccines we have, and when each one was introduced.1
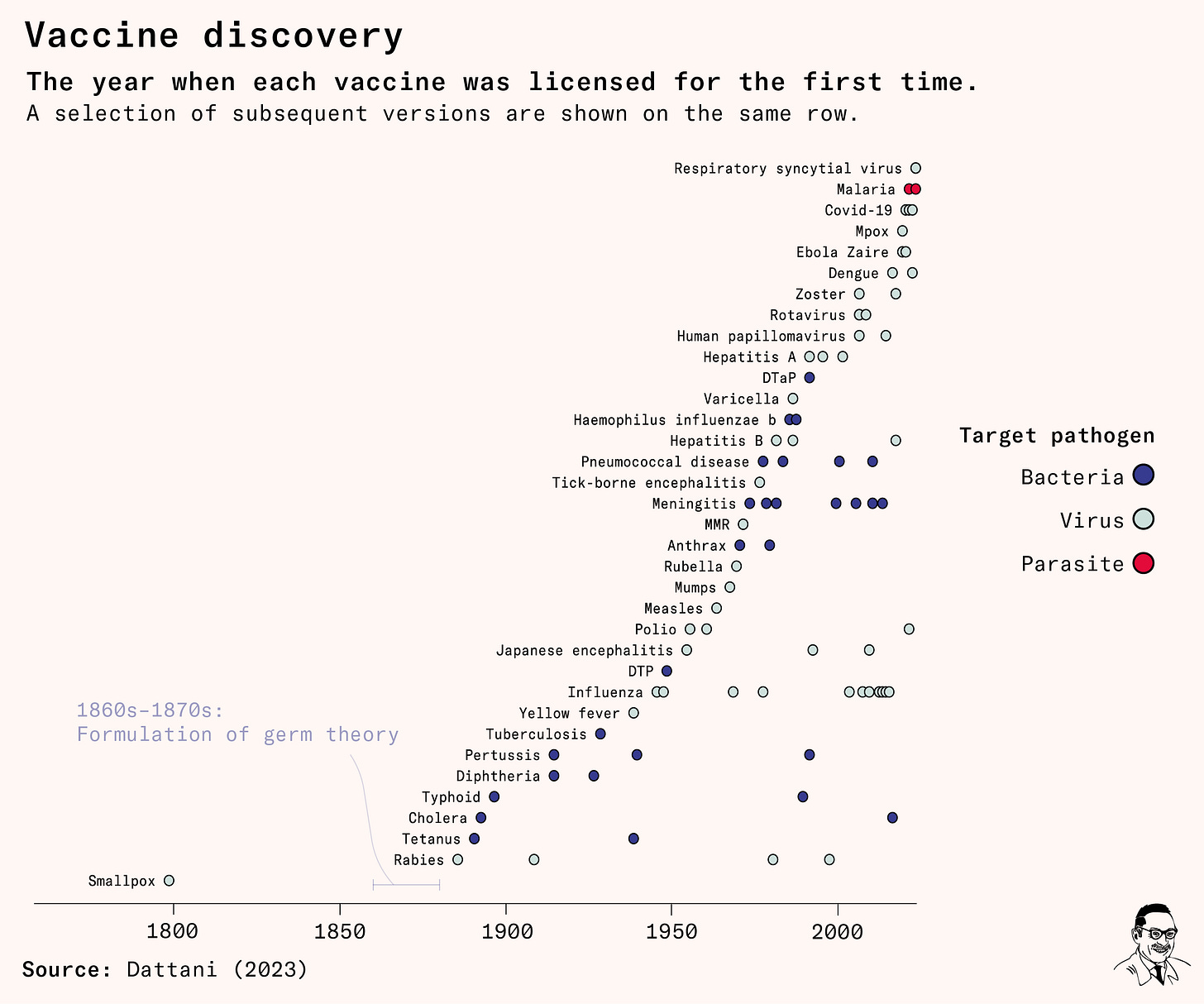
The malaria vaccine licensed in 2021 is the first vaccine against a parasite in humans. (There had already been numerous parasite vaccines for livestock and pets.)
It’s also striking to see the long 80+ year gap between the first and second vaccine. When Edward Jenner developed the smallpox vaccine, he didn’t know it was caused by a microorganism – let alone a virus – because germ theory hadn’t developed yet. When it was, vaccine discovery finally took off.
Even with germ theory though, viruses were too small to be seen under microscopes at the time. The first virus was observed all the way in the 1930s. This also seems surprising from the chart. Viruses were theorized – and extracted, purified, named and used to develop vaccines – before they were observed.
To develop the 1885 rabies virus vaccine, for example, Louis Pasteur and Émile Roux isolated an unseen material from the spinal cords of rabid animals. They purified it by passing it through thin porcelain filters that would remove bacteria, which are much larger.

In this post, I’ll add some thoughts on what it was like to work on a 9,000+ word article; how to fact-check historical claims; and a few more bonus things I learnt about the story that didn’t quite fit in.
How to write a 9,000+ word piece
First, make sure you have a couple of great coauthors and editors. I’m only half-joking. I wrote the ‘science and history’ part of this piece, while my coauthors wrote about the economics and policy ideas. So it would’ve been very different and lacking in ‘so what?’ if I’d written this alone. And my colleagues Ben and Sam are fantastic editors whose questions really strengthened the piece. It was a real team effort.
Behind the scenes though, this was the 5th or so complete rewrite of the piece. It’s such a hugely important story, and such a complicated one, that it was hard to figure out how to cover everything and make it very readable and hopefully enjoyable.
Here are a few things that helped.
Tell the story to people, in-person.
By the second or third redraft of my part, I felt pretty stuck. There were so many different aspects that delayed the malaria vaccine, that I couldn’t figure out how to arrange it all in a way that made sense and was easy to follow.
So I had a half-hour roundtable conversation with some friends and writers who don’t have a science background, and told them about the story and what I was struggling with.
It surprised me which parts they found surprising – that malaria was caused by a parasite, not a virus, and why this would make a difference. Or, how animal models and challenge trials worked. So I decided to spend more time elaborating on these parts.
Explore, ask, and write a list of things you don’t yet know.
One of the biggest struggles I had was that I’d tried to write my part of the piece while I still had unanswered questions. There were so many different rabbit holes I could’ve gone down and started working into the piece, but only some would actually be relevant.
First, I just vaguely explored the topic – I read existing articles and reviews on malaria vaccines, and formed a basic understanding of the problems.
Then I talked to several experts with questions I still had. Talking to experts isn’t necessarily about getting precise answers – conversations are rarely so detailed. But I found it very useful to hear pointers on what I should be looking into.
For example, some experts explained that adjuvants were critical in making malaria vaccines effective – so I read much more about them. Some mentioned the WHO’s decision in 2015 to ask for more pilot studies – so I dug into what happened then.
Every expert I spoke to said that the main reason we didn’t get a malaria vaccine sooner was because it primarily affected the poor. This was painful to hear; it was also a big motivation for me to find out the details and get this piece finished (which we’d been working on for several months).
At this point I tried writing my part of the article. It still didn’t work – my writing went into dead-ends; I over-explained some parts, while not knowing enough about the others, and often had no idea how to progress from one section onto the next one.
Sometimes the most ‘obvious’ structure of a piece is the best one.
It didn’t occur to me until quite late that the best way to write this article would be chronologically. In retrospect, that seems obvious and natural – but it wasn’t at the time.
Because the answer to the question has so many layers, I eventually realised it would be logical to build up the piece progressively, chronologically, as a story that developed as scientific understanding grew – first, with the basic facts, and then with the major complications that people faced, in order.
This meant the piece needed much more historical context than I initially expected, to follow along the scientific story. Still, it made the piece more interesting than it would’ve been otherwise.
List questions and find out what you still don’t know.
At this point – still fairly stuck – I wrote down a list of questions I still hadn’t answered.
For example: Why did the malaria eradication program fail? When did DDT resistance evolve? Why did the researchers pick the CSP protein for the subunit vaccine? How did malaria vaccine funding change over time? How many malaria vaccine trials had been ongoing since 2001? What are the barriers to doing clinical trials in sub-Saharan Africa? How were the sequential trials done?
Then I closed the tab with my google doc draft, and kept it closed for days, while I went and tried to find the answers to each of them. I wrote notes with links and references below each question I’d had.
Now, finally, even the parts that weren’t in my memory were at least written down accessibly. I could finally start writing the piece properly and fill in the remaining details, fact check and adjust anything afterwards.
Fact-check as much as you can.
The worst thing about ‘fun facts’ is how many of them are not facts at all. They often come out of misunderstandings, misrepresentations, or simply trace back to nowhere. Zombie anecdotes and statistics are very common, including in academic reviews by subject-matter experts.
In my view, it’s not surprising that this happens. We misremember numbers and details. We like fun and interesting stories, and we don’t have inherent reasons to distrust popular historical anecdotes that lots of people believe.
But this means errors compound over time. It’s easier for science to be correcting when we continue to face the same problems, and accurate details of a specific concept continue to be important – like knowing how many genes are in the human genome. This seems less the case for historical anecdotes; they seem more like the game telephone (or ‘Chinese whispers’), where people insert new misunderstandings into the story as it gets passed along over time, and people cite newer and newer reviews on the topic, rather than primary sources.
I learnt a lot about how to go about fact-checking historical claims from talking to the historian of innovation Anton Howes, who is also a great friend of mine. He recently wrote a fantastic post after our conversation called ‘Does history have a replication crisis?’ which I really recommend.
What helped?
Tracking down & citing the primary source if I could.
Not a review from the 2000s – if something supposedly happened in 1956, I should go and find a reference from then, from official sources or the person involved in the research themselves. Google Scholar and Archive are really helpful for doing this – you can filter search results by year, and actually read documents from the time.
It’s often fun, inspiring and eye-opening. Biology papers from a century ago are surprisingly perceptive, and help you place yourself at the time – you learn what people struggled with, which technologies they had access to, and which unanswered questions they had. This was also really helpful to write the piece in a style that brought people along on the journey.
Here, for example, is a book (in French) by Charles Laveran on his research on the parasite that causes malaria. I searched through this and other documents from the time to find out whether it was really true that he showed Louis Pasteur and Robert Koch a sample under a microscope in person and immediately convinced them, as one review put it (without a citation). But I found absolutely nothing documenting this from the time. So I removed it, along with anything else that seemed unsupported. If we really want to understand what problems people have faced, to learn from them, accuracy is far more important than a fun story.
Finding niche, in-depth reviews of the precise topic, with precise details.
When I wanted to learn about the story behind how the rodent models were discovered, I read a book called Rodent Malaria.
Other sources about animal models lacked citations and details, and also misrepresented the story – they made it sound like rats affected by malaria were found immediately after researchers looked for them in the Congo. Instead, the book explained that it took two years to find one: researchers had tested hundreds wild rats in the Kisanga province, until they found one positive for the malaria parasite. I then tracked down the original bulletin and paper describing the finding, by searching for the authors and restricting the time-range, which corroborated this.
I think this is a useful ‘red flag’ to recognise a zombie claim – it lacks names of specific people, places and dates. That’s not always good enough, because specific names might just be specifically wrong. Still, this heuristic is useful to know when to dig into claims that could just be hearsay, that lack details or citations because the person doesn’t know them.
I went into a similar rabbit hole trying to trace the story of ‘the cobra effect’ a while ago, and wrote a thread about it here.
Corroborating the narrative. Does it make sense, given what else you know about the time?
Take the finding that DDT was harmful to animals, birds and fish. It’s often thought that there wasn’t research into this until the 1950s or ‘60s, or until Silent Spring was published in 1962.
However, I found quite a few papers describing its harms from the 1940s – this review from 1946 had a great summary of what was known at the time. It makes a lot more sense considering the pesticide was an insect nerve poison developed during World War Two, and much of the early toxicity research was conducted by the US army.
It’s a shame that all this was necessary, as it’s very time consuming. In ideal circumstances, we’d be able to outsource our understanding of specifics to other people with expertise. Anton had some great ideas on how to fix this problem, but it’s likely there’s a lot of different ways to improve the situation.
What I learnt about scientific discovery
I used to find it a little funny that this blog was called ‘scientific discovery’ even though not much of it focused on the process of discovery, or the history of fields I was interested in. From now on, I’d like to make that a much bigger focus.
I think the buzz around the ‘room-temperature superconductor’ is an example of how the science-hype cycle is often coverage of speculative technologies that might become practical years from now, assuming they work out at all. Meanwhile, there are just passing mentions of breakthroughs that have finally succeeded and why. There are rarely retrospectives on how much they’ve made a difference to people’s lives.
Both successes and failures can be incredibly informative. Why did some ideas work but not others? That sounds like a fuzzy question that’s difficult to answer, but if you look closer there are often very specific obstacles that scientists faced – that needed to be overcome, or avoided in some way.
This piece – on the story of the malaria vaccine – is about an enormous achievement, but it’s also about many disappointments and failures along the way.
Here are some things it demonstrated to me about the process of discovery.
Iteration. Parts of the story show how discovery is often iterative, once the ‘active ingredient’ is known. Once chemicals with a particular structure had been discovered as insecticides, several more were identified, including DDT. After one vaccine with the CSP protein was shown effective, another successful one was developed with a similar formulation.
Non-linearity. The process of discovery is not necessarily linear, as Jason Crawford has written previously. It doesn’t always go from basic research to engineering, but goes back and forth between tinkering and genuine understanding.
The first vaccines were developed before the development of germ theory, and before the discovery of viruses. But once this knowledge had grown, with the discoveries of specific pathogens and how they caused disease, it finally led to a stream of new vaccines.
Multiple causes. The story shows how the same steps could have been sped up in multiple ways. The discovery of a useful animal model could have been found through closer attention to the original findings; or if more researchers had been working on the problem; or pure luck, stumbling upon a more practical animal model first.
This was also true of the final part of the journey – malaria trials could have been sped up if there had been more existing trial infrastructure in sub-Saharan Africa, if there was more philanthropic funding, with Advance Market Commitments, and so on. As we explain in the piece, AMCs are one of the best ideas in this situation because they get the technology past the finish line and help scale them up.
I also really liked this thread by Jacob Trefethen on other policy ideas and implications from the story.
People, goals, times and places. Fundamentally, the malaria vaccine story is about how discovery doesn’t happen in the abstract, but is driven by people motivated to work on the problem, who live in a particular time and place. They’re limited by their knowledge and access to technology and resources, and influenced by the economy, international efforts and programs, and sometimes even war and conflict.
And that’s all! I hope you enjoyed reading this and the piece, of course. Here it is again if you’d like to read it now.
I’ll be back soon with a roundup of great new scientific research I’ve been reading, so I hope you’ll subscribe if you haven’t already.
See you next time :)
– Saloni
Thanks very much to Jacob Trefethen for pointing out that a newer dengue vaccine was recently approved. I’ve updated the chart and article to reflect this.


This was really a fantastic article. Thanks for all your hard work.
Wonderful piece. Thank you for giving us some insight into the behind the scenes. This has been useful for my own research.