#13: The success of chickenpox vaccines
This week: Why not treat chickenpox and shingles as an avoidable problem? Plus, I have a PhD now.
This is my thirteenth post of Scientific Discovery, a weekly newsletter where I’ll share great new scientific research that you may have missed. Check out the About page if you’re interested in why I’m writing this.
Hello again! I passed my PhD viva two weeks ago! I’m very happy about it and I guess I’m officially a “Dr.” now, which feels strange.
But I also now have a long backlog of posts I want to write, so I’m going to get back into it. If you like this, I hope you subscribe! And if you spot any errors, please let me know so I can fix them.
The untold success of chickenpox vaccination
In most of the world, if you’re older than twenty, you probably remember having chickenpox as a child. It’s estimated that 85–90% of people have had it by the age of 15.
If you’re older, you might have also had shingles – a painful condition caused by the same virus getting reactivated later on.1 The virus lays dormant in the periphery of the spinal cord, and can reactivate months, years or decades later, resulting in rashes, an itchy burning sensation, and in some cases, long-term nerve pain.
But people tend to think of chickenpox as just a mild disease or a ‘rite of passage’ for children. Chickenpox vaccines are often considered not worth the cost, and some people worry that vaccination during childhood will be pointless, as it will simply delay disease until adulthood when the disease is more severe.
The last few decades have seen substantial evidence against these ideas, and I think they have a story that everyone should know.
Chickenpox vaccines were first developed in the early 1970s by a Japanese virologist named Michiaki Takahashi, and went through testing and further development over the next decade. They were first licensed in Germany and Sweden in 1984, and in the US in 1995.
They are highly effective. With two doses, they reduce the chances that a child would get chickenpox by 95%, or severe disease by 99%, over the next 10 years.
In 1995, the same year they approved the vaccine, the United States Centers for Disease Control and Prevention (CDC) made the then-controversial decision to offer the vaccines routinely for all children at no cost. It was the first country to do so, and as a result has the longest experience with it.
Four years later, chickenpox vaccination became required for entry in childcare and school, with some medical and religious exemptions. [Since 2005, chickenpox vaccines don’t have to be given as an additional injection, because some formulations include it within the MMR vaccine, as an “MMRV vaccine”.] From 2007 onwards, a second dose was recommended and routinely given out as well.
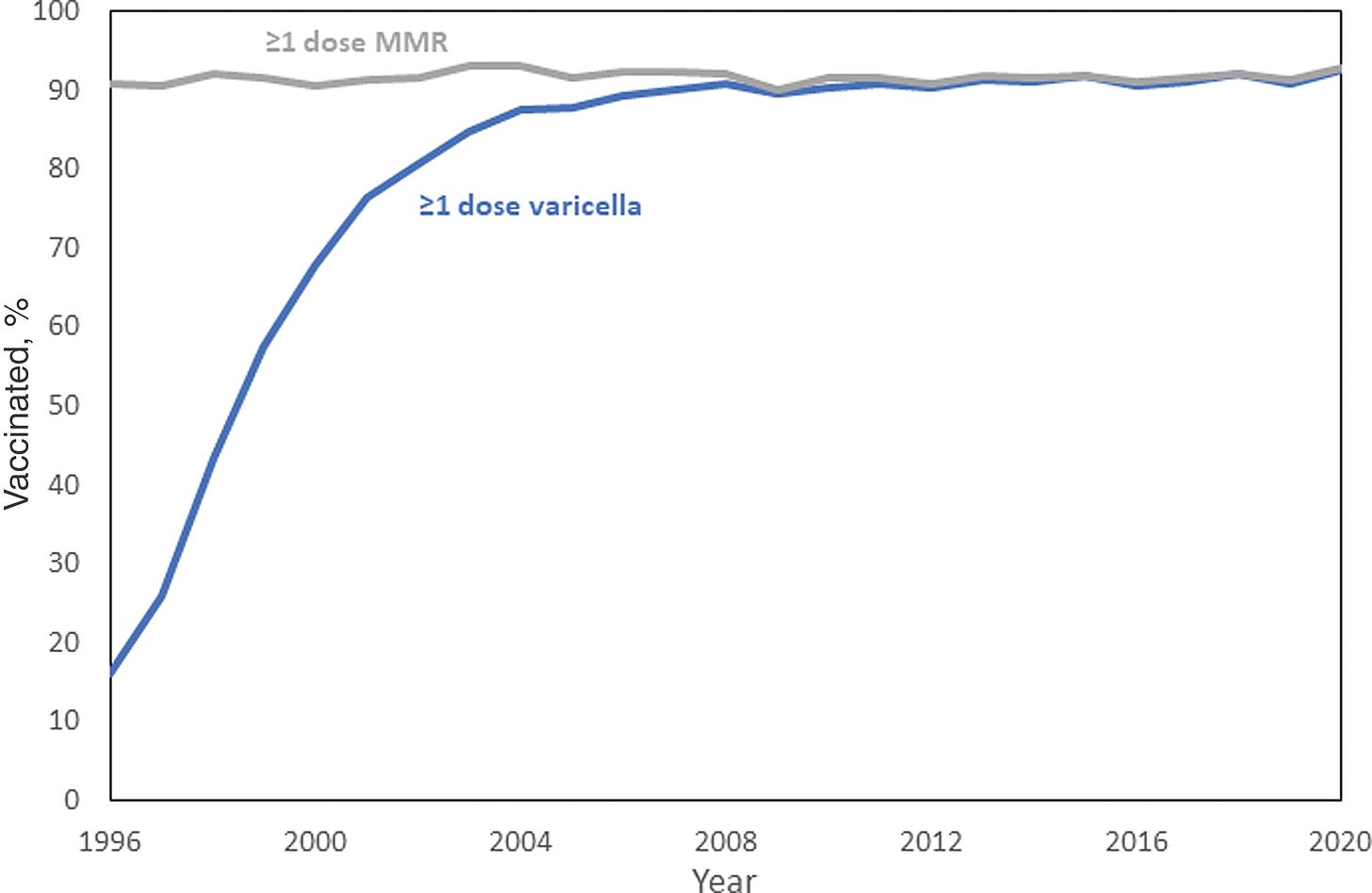
Before vaccination, chickenpox was common, usually painful, sometimes severe, and a heavy burden on parents taking care of children at home.
The worst outcomes were rare, but visible because the disease was so widespread. Around 5 in 100,000 infants and 1 in 100,000 children who caught the disease then died because of it – it could cause secondary infections and pneumonia, neurological complications and haemorrhage.
Children who died tended to have immune conditions, or had leukaemia and were on track to be cured by highly-effective chemotherapy. Some weren’t recommended to take the live vaccine and had to pause treatment while they took it, or simply had to try avoiding the virus from other children and adults, which I’d imagine is pretty hard when chickenpox is so common.
In the early 1990s, before the vaccines were introduced, the virus caused around 4 million cases of disease, 12,000 hospitalisations and 100 deaths each year in the United States. Children were most affected, making up >90% of cases, >60% of hospitalisations and around 40% of deaths.
I’ve used past tense, because here’s what happened after that.
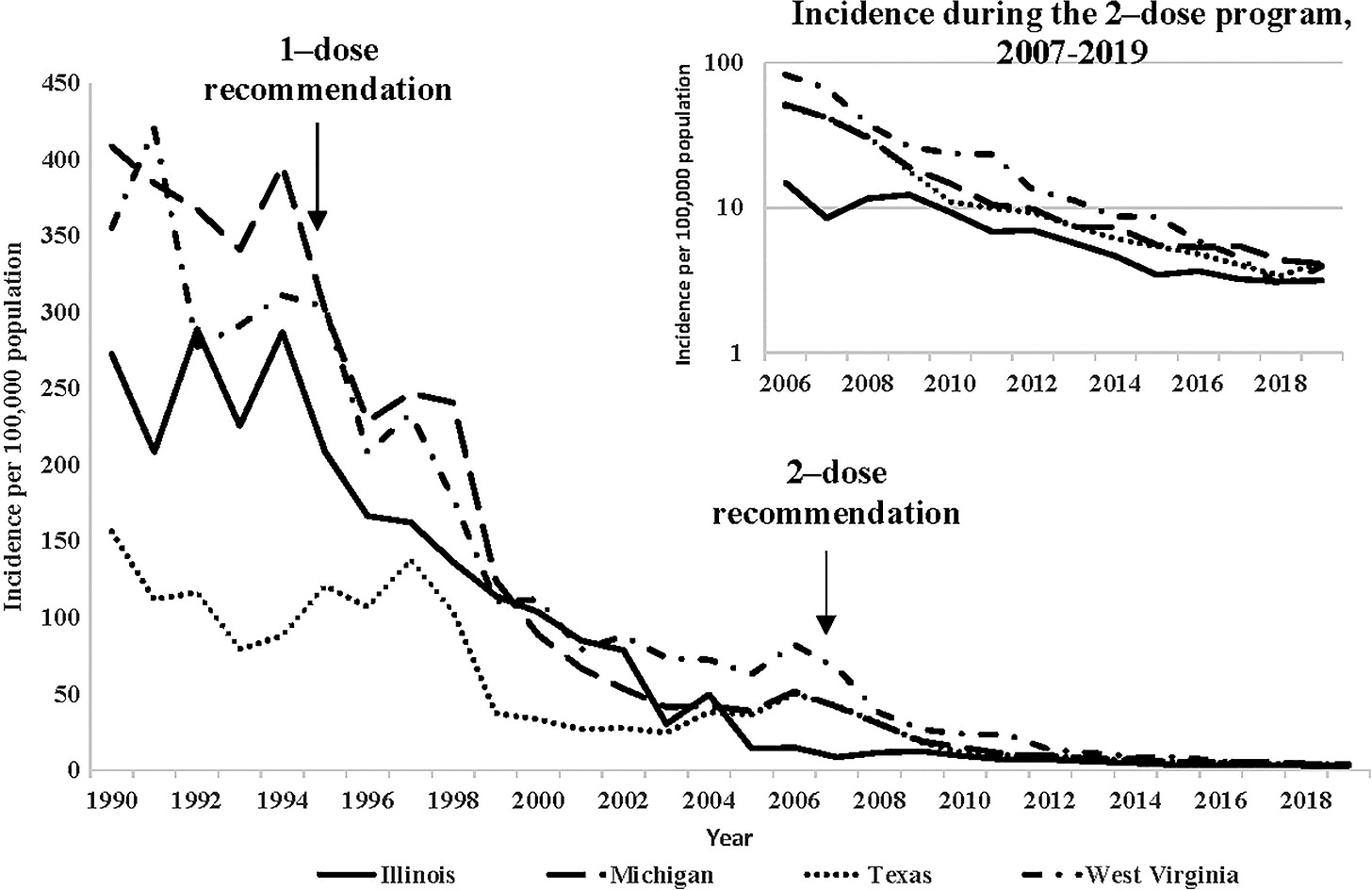
25 years after routine vaccination in the United States, rates of chickenpox had declined by over 97%. Hospitalisation rates declined by 98% in children and 84% in adults.
Deaths, which were rare, declined by 93% among people aged under 50. In under-20s, they shrunk to zero. Chickenpox was eliminated as a cause of death in this age group.
And despite the worries, they didn’t worsen the burden in older age groups.2 Instead, adults also benefitted from the reduced spread of disease among children, and were less likely to develop the disease, be hospitalised or die from it.
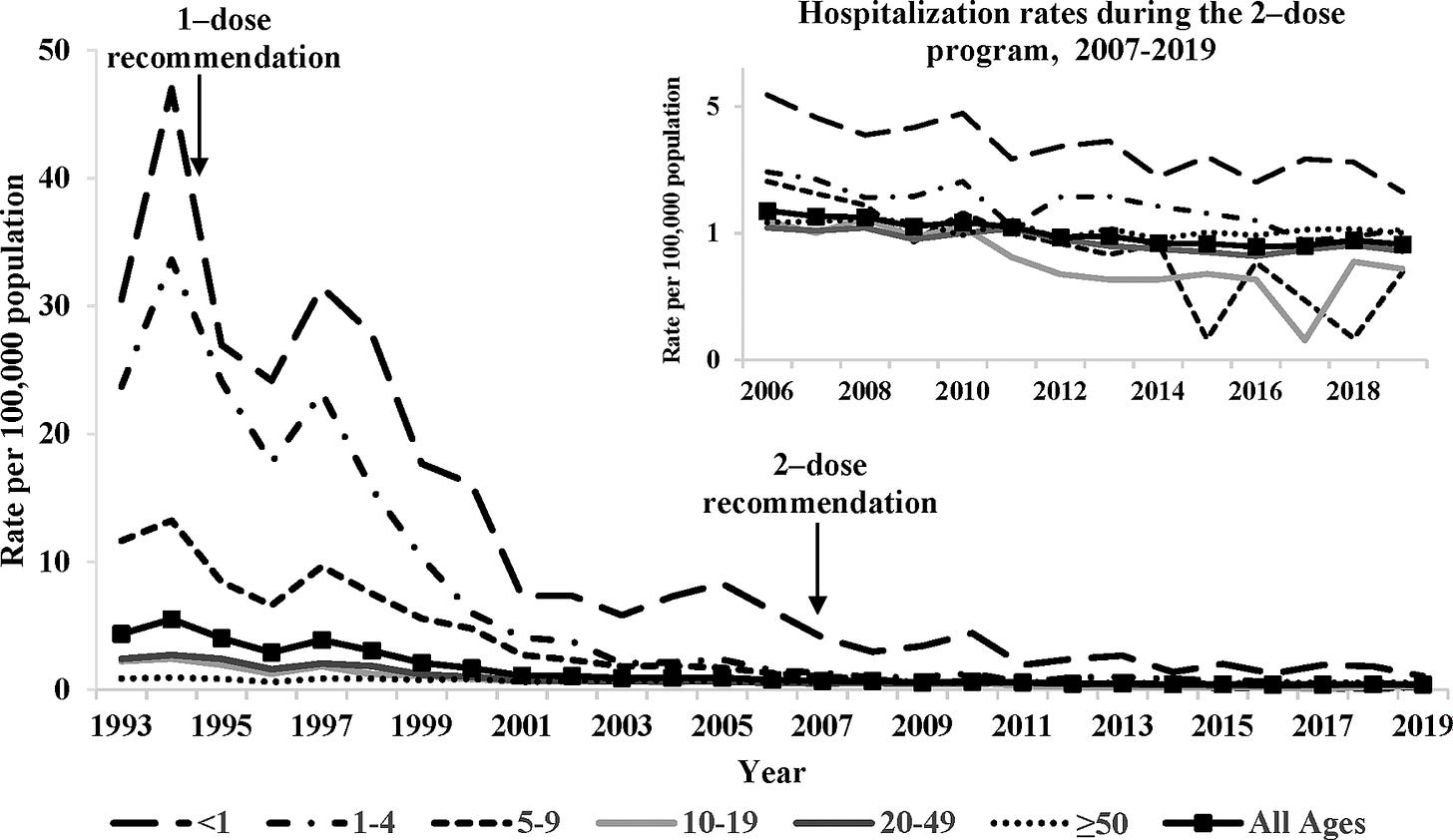
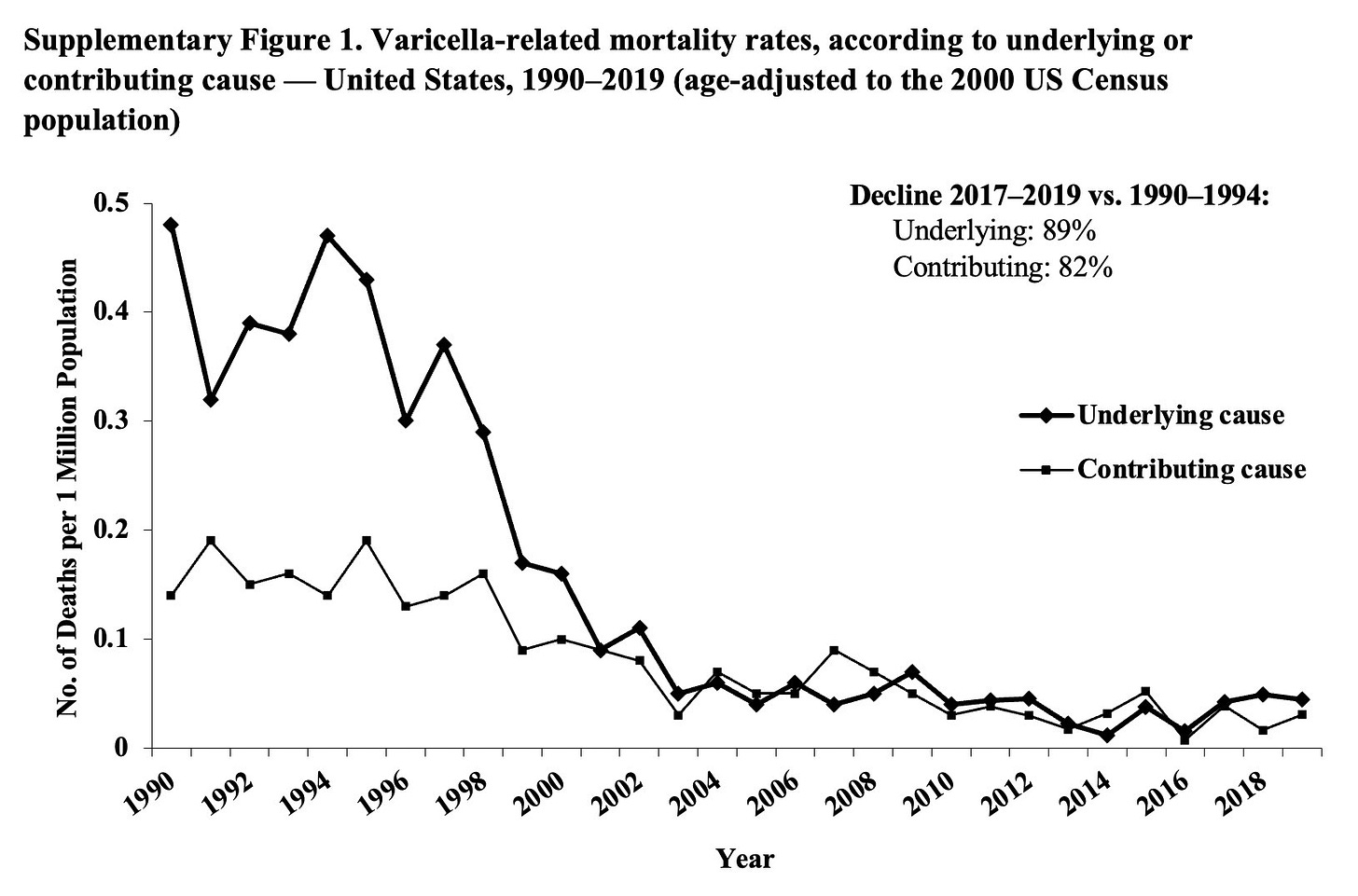
In the 25 years since vaccines were routinely provided, it’s estimated that a total of more than 91 million cases of chickenpox, 238 000 hospitalizations, and almost 2000 deaths were prevented in the US, as a result of vaccination.
The decision was also cost-saving: this conservative estimate puts it at a net societal savings of 23.4 billion US dollars so far, with savings coming from the reduction in sickness, fewer work-days lost to childcare, and avoiding expensive treatment including antibiotics and antivirals for other infections that could arise as complications of the disease. That is, chickenpox vaccination also reduces the need for antibiotics, and thus helps against antibiotic resistance.
With a simple calculation, each case of chickenpox averted saves around 260 US dollars on average.3 This includes the costs of producing the vaccines, administering them, the potential of side effects, and the time and cost taken to travel to get vaccinated.
In the years to come, the total benefits of the decision will grow, as more children avoid the earlier levels of spread, and as the herd immunity that has built up continues to benefit future generations.
America’s success with chickenpox vaccines isn’t unique. Many other countries – including Japan, Canada, Germany, Brazil, Australia, Costa Rica, Taiwan, Uruguay and Qatar, among others – have also routinely offered vaccines to all children at no cost for years and seen big successes.
Rather than worsening disease in older people, they’ve seen the opposite – huge declines in rates of hospitalisation and deaths from chickenpox across age groups.
But many countries around the world do not yet offer chickenpox vaccines routinely4. Here they are on this map, in yellow.
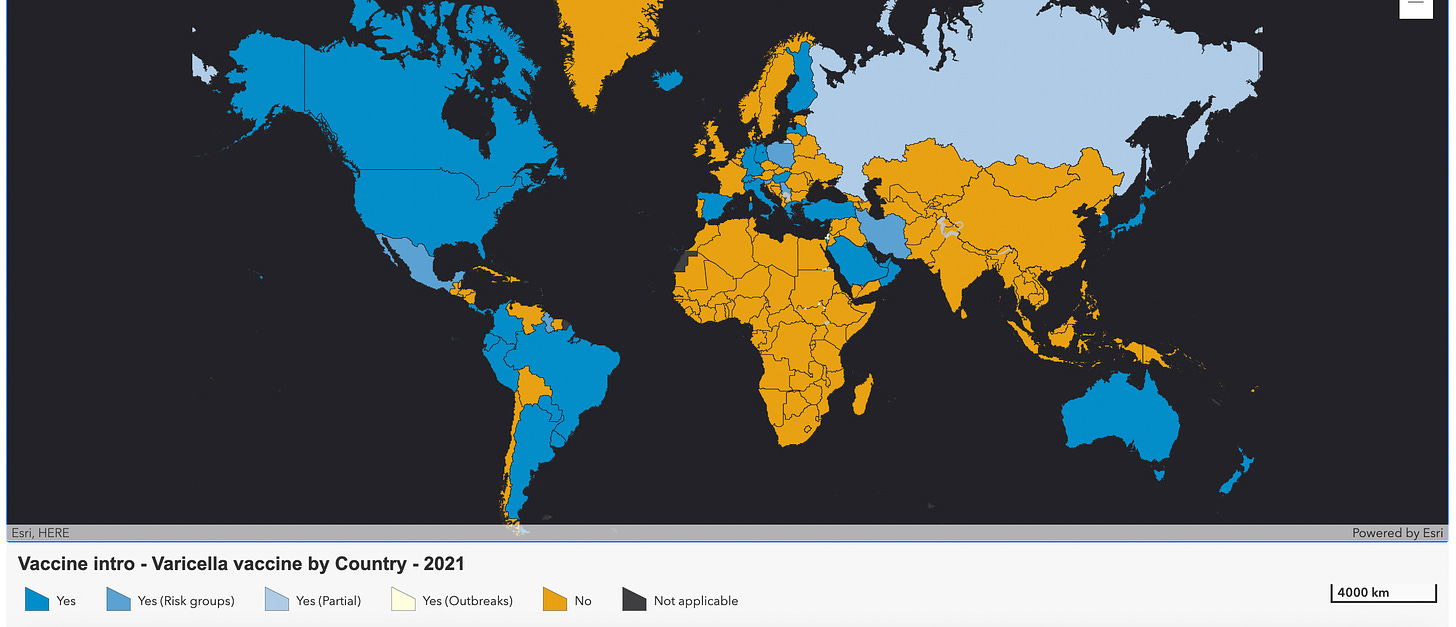
As I mentioned earlier, chickenpox is just the first part of the problem.
Around 30% of people develop shingles in their lifetime, from the same virus that had given them chickenpox years before. Shingles causes an itchy burning sensation, rashes, and can lead to complications with vision.
You can see the per-year rates of developing shingles in the chart below. The rates are low early in life, but start to grow quickly around the age of 45.
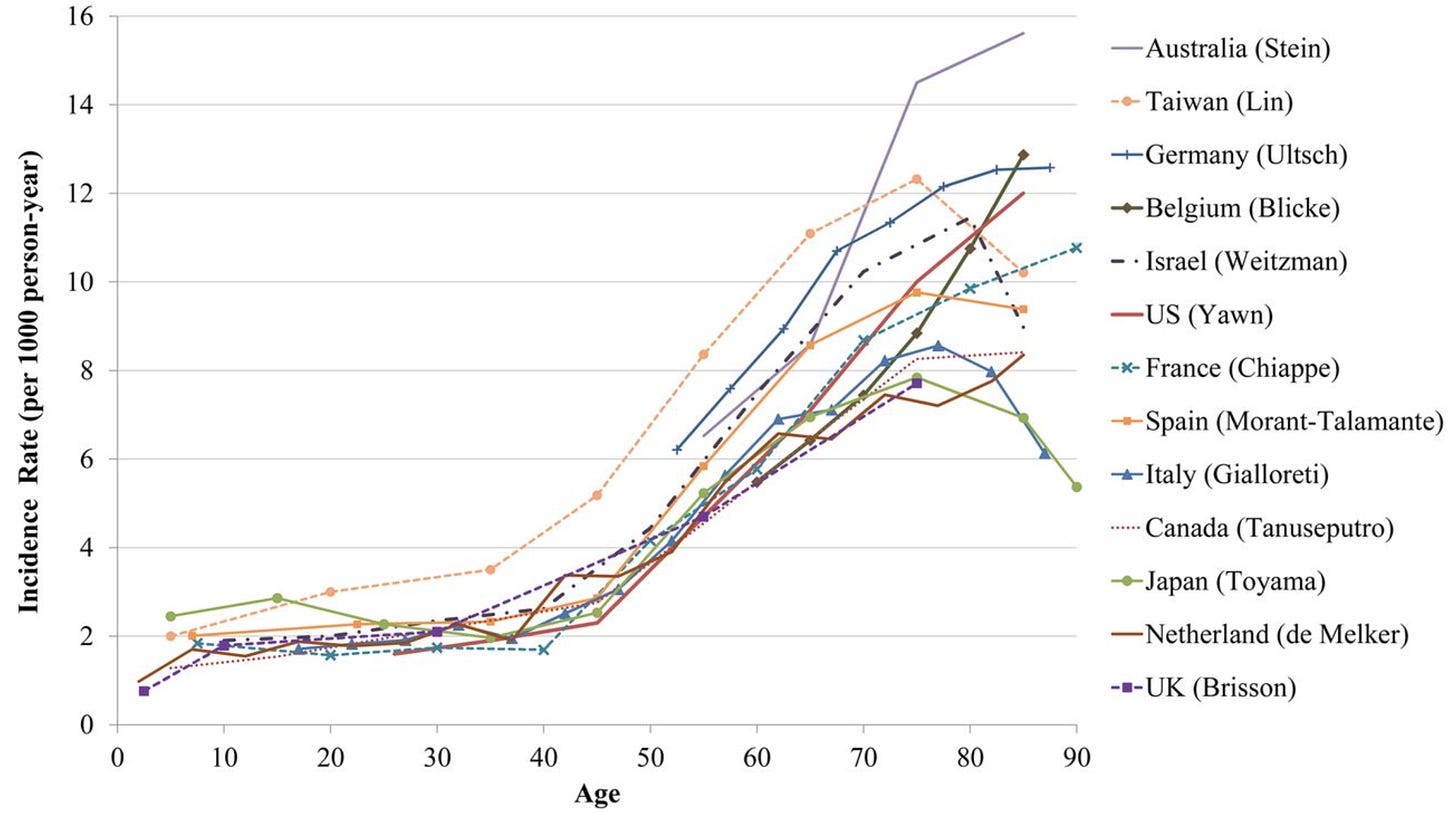
Shingles can also lead to long-term nerve pain, called “post-herpetic neuralgia”. People experience this differently, but it can feel like either a continuous burning sensation, or recurring electric shock-like pains, or pain in response to touch.
How often does that happen? This systematic review estimates it happens in around 5–30% of people in their lifetimes, and that 30–50% of them continue to experience it for at least a year. Here’s a chart that shows this with data from the Netherlands.
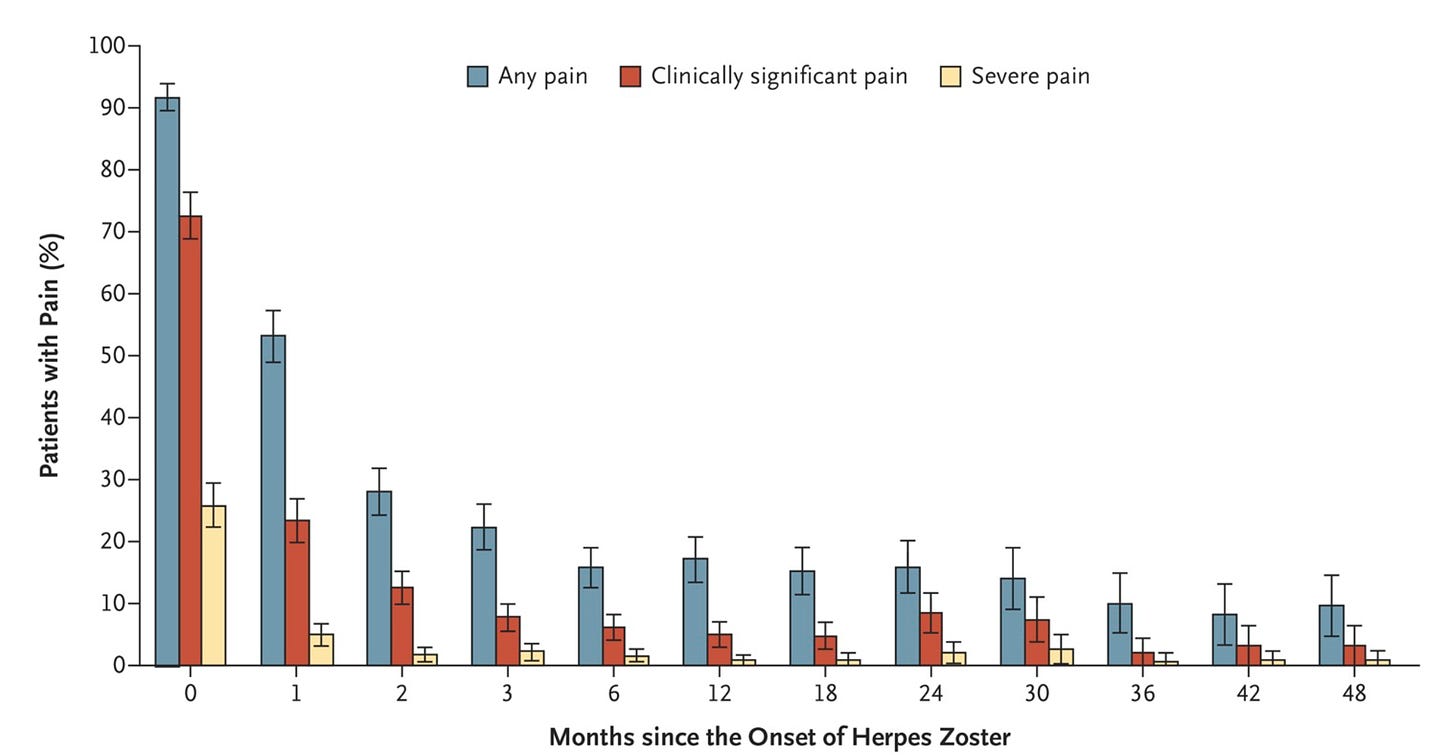
If you’re like me and weren’t vaccinated for chickenpox as a child and caught the disease, you can hopefully get a shingles vaccine, which reduces the risk of shingles by >90%. In many countries, it’s available after the age of around 50. An older version of the shingles vaccine contains the same formulation as the chickenpox vaccine, but at a higher dose; while a newer one is a recombinant vaccine.
It’s maybe not surprising that America’s chickenpox vaccination programme reduced shingles as well, since it’s caused by the same virus reactivating. Before it, rates of shingles had been growing in the US for decades. After it, the growth began to slow down, plateau and eventually fall across age groups starting with the youngest.5

In total, the routine vaccination programme was highly successful in many ways. It massively reduced the rates of chickenpox, and hospitalisations and deaths from the disease. It resulted in a net societal savings, and likely helped against the development of antibiotic resistance to other diseases often affect people with chickenpox. And it slowed down the rates of shingles, which had been growing.
(The cost-effectiveness estimate I described above doesn’t include the effect on shingles, which means it underestimates the benefits.)
At the start of this post, I didn’t even describe the typical symptoms of chickenpox. I assume most of you have memories of what it was like – at least I do. I remember having it while visiting my family in India on vacation, which I just had to spend at home with incredibly itchy rashes across my body and face, while I kept vomiting for a week.
Younger generations won’t remember anything like this, I hope. But what it will take to get there is our generation treating it like a problem that can be avoided.
I hope you enjoyed reading this! I’ve proofread and double-checked numbers, but if I’ve made any errors, please let me know.
Next week I’ll be back with another post about one of the most underrated topics in global health – missing data. That’s based on a talk I gave at EAGxCambridge on Saturday (slides here). Eventually, I’ll also get around to catching you up on other stuff you missed since December.
See you next time!
– Dr Saloni (haha)
Under the hood
Here’s something new I’m trying – explaining the data quality and assumptions underlying these studies and sharing my reasoning on this topic.
I hope this helps other people learn how to read studies and evaluate their quality, or at least know what I look out for. And I also hope it helps me hold myself to a high-standard, and that it allows you to see if I’ve made any mistakes.
Let’s talk first about where the numbers come from.
I’ve cited many studies in this post. The main focus is the decline in cases, hospitalisations and deaths from chickenpox in the United States.
For cases, the data comes from 4 states which had surveillance before 1995, when routine vaccination began. Although these states may not be representative of the prevalence across the country, their data is useful because other states lacked consistent surveillance beforehand and hence don’t allow for an easy comparison of before and after. Other states have consistent data from 1995 onwards, as part of the ‘active surveillance’ that began with the routine vaccination programme.
For hospitalisations, the data comes from the ‘National Inpatient Sample’, a very large nationwide dataset that includes about 20% of all discharges from hospitals in the US. It’s nationally representative in terms of a range of hospital characteristics, such as the number of beds, the urban/rural location, type of ownership and several other factors.
For deaths, the data comes from the ‘National Center for Health Statistics’, which includes deaths across the entire population in the country, using data from death certificates. The data includes deaths categorised by their ‘underlying cause of death’ and other contributing causes of death listed on the death certificate; I’ve included charts showing these separately and together in the post.
The cost-effectiveness analysis looked at a range of scenarios, to derive conservative estimates of the net costs of the vaccination programme even if the estimates used to derive it were mistaken. This included varying the rates of incidence, hospitalisation and mortality, the costs of each case, the costs of vaccine administration, and the costs of side effects; each by +/-20%. It also looks at a worst-case scenario where each one is 20% worse – even in this worst-case scenario, the savings are estimated to be 9.2 billion. The range of estimates are shown in the paper. (Note that the authors exclude the effect of vaccines on shingles, which is one reason why they are an underestimate of the savings.)
Aside from those studies: Data on efficacy comes from RCTs that compare chickenpox vaccines to MMR vaccines (which are an ‘active placebo’ for chickenpox vaccines). Data on lifetime rates come from seroprevalence studies for chickenpox (since case-surveillance only happens in a few countries), and large longitudinal studies for shingles. I’ve also used multi-national systematic reviews for other estimates when I’ve found them, to avoid picking data from individual countries, in case there’s a lot of variation and I happen to pick an odd estimate.
Second, how much of the decline should we attribute to the vaccines?
Actually estimating this is tricky, but there are things we can say.
The efficacy rates of these vaccines are very high – around 95% against chickenpox and 99% against severe disease over the next 10 years – and the vaccination coverage in the US was also high – around 90% among infants from 2006 onwards. Together, this is a simple benchmark that implies the impact could eventually result in the entire decline. (The speed of this impact would depend on how quickly those rates are achieved across age groups who account for the hospitalisations and deaths.)
But there are other things to consider as well:
The efficacy figures don’t account for the effects of herd immunity – higher vaccination rates also reduce the transmission of the disease, which means the reduction in severe disease could be greater than a simple calculation of efficacy multiplied by vaccination rate.
The median estimate by the CDC researchers assumes that the rates in 1990–95 would have remained at that level, without the vaccines. But the rates of shingles, which track chickenpox because they’re caused by it, had been rising across age groups in the US before the chickenpox vaccination program.
Unfortunately, before routine vaccination for chickenpox, there hadn’t been national surveillance for chickenpox cases in the US, so data on cases before that comes from only a few states, shown in the first chart.
It’s also worth noting that, in other countries that don’t have routine vaccination but do have data, including the UK, cases of chickenpox and shingles have continued to rise over decades, especially among older age groups. This heavily implies that you wouldn’t see such a large decline due to other ongoing factors.
In the US, shingles cases have declined in the youngest, and risen but slowed down in older age groups who aren’t eligible for shingles vaccines.
Overall, there’s a lack of global data on this topic, and data is most-often missing from countries that don’t have routine vaccination programs.
An alternative is to use an epidemiological model – which could account for the ‘herd immunity’ generated by vaccines. But these can depend heavily on estimates of the transmission reduction, the duration of immunity, levels of contact between people, and other factors which are often hard to estimate precisely. You might be interested in these other studies that make these estimates for some other countries.
So, while other hypothetical factors could aid the decline too, I think the vaccines can account for most or all of the decline.
Here’s a little bonus, in case you’re interested.
In the 1960s, a scientist named Robert Edgar Hope-Simpson discovered that chickenpox and shingles were caused by the same virus. How did he figure that out? You can read the whole explanation in a lecture he gave in 1965 here.
It’s quite readable – and as someone that spends time thinking about how people can identify cause and effect without RCTs, or were able to even before they existed, I enjoyed it.
Update 20/3/2023: I added a couple of lines to clarify that the cost-effectiveness analysis didn’t include the effects of chickenpox vaccination on reducing shingles, which means it underestimates the benefits.
I corrected a description of the shingles vaccine, which mistakenly said that it had a lower dose than the chickenpox vaccine. I also added links to learn more about the shingles vaccines.
In medical literature, chickenpox is usually called ‘varicella’. Shingles is either called ‘zoster’, ‘herpes zoster’ or ‘varicella zoster’. Both chickenpox and shingles are caused by the same virus: varicella zoster virus. In this post, I’ve just stuck with calling them chickenpox and shingles, since those terms are familiar to people.
One reason that ‘age shifts’ may appear at first is that cases had been growing across age groups before vaccination and continue to rise in older ages while slowing down and declining more quickly in children. Other times, these are actually shifts in the proportion of all cases that affect older people, even when the rates decline across each age group.
Even though they can be given within the same formulation as MMR vaccines, therefore even avoiding the need for an extra injection.
In older age groups who aren’t eligible for shingles vaccines, and missed chickenpox vaccines, cases had been growing rapidly since much before the vaccination programme, but have recently begun to slow down.



Very informative! Many congratulations on your doctorate!
Chicken pox vaccination is one of my gnawing bad consciences. I live in a country where the vaccine is not offered without cost so I let my children be contagiated at low ages in daycare. Not optimal - maybe I could get the energy to search out the vaccine for my youngest child, who hasn't caught the disease yet. It is always easier when vaccines are offered for free within the frame of the ordinary vaccine program.